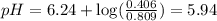Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, alfarodougoy8lvt
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 01:30, giraffegurl
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3


Chemistry, 22.06.2019 08:30, melikefood01
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1
Do you know the correct answer?
What is the pH of a buffer prepared by adding 0.809 mol of the weak acid HA to 0.406 mol of NaA in 2...
Questions in other subjects:


Mathematics, 23.07.2019 23:00

Mathematics, 23.07.2019 23:00


Biology, 23.07.2019 23:00




History, 23.07.2019 23:00


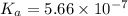
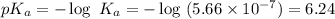
![ pH=pK_a+log\frac{[salt]}{acid]} ](/tpl/images/0539/5199/2cb89.png)