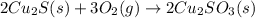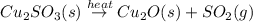Chemistry, 10.03.2020 00:40, leysirivera23ovez6n
When copper sulfide is partially roasted in air (reaction with o2), copper sulfite is formed first. subsequently, upon heating, the copper sulfite thermally decomposes to copper oxide and sulfur dioxide. Write balanced chemical equations for these two reactions

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, genyjoannerubiera
Si una estrella no tiene paralaje medible, ¿qué puedes inferir?
Answers: 1

Chemistry, 22.06.2019 06:30, cadenhuggins2
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 10:50, adam1299
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments, solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1
Do you know the correct answer?
When copper sulfide is partially roasted in air (reaction with o2), copper sulfite is formed first....
Questions in other subjects:

Social Studies, 05.03.2021 21:50



History, 05.03.2021 21:50


Spanish, 05.03.2021 21:50

Spanish, 05.03.2021 21:50

Chemistry, 05.03.2021 21:50


Mathematics, 05.03.2021 21:50