Chemistry, 10.03.2020 00:37, serenityarts123
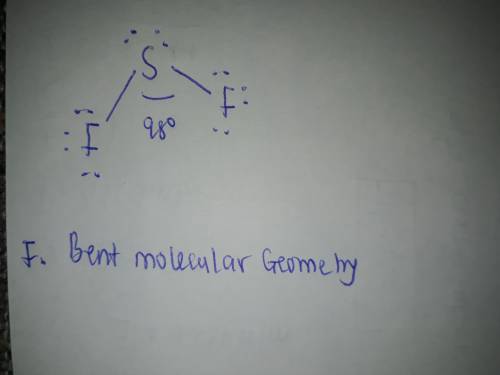
Draw the Lewis structure of SF2, showing all lone pairs. Identify the molecular geometry of SF2.
A. square pyramidal
B. trigonal pyramidal
C. trigonal planar
D. linear
E. T‑shaped
F. bent
G. square planar
H. octahedral
I. trigonal bipyramidal
J. tetrahedral

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 12:20, hayleighhurt
Describe the structure of ammonium lauryl sulfate. refer to the given diagram. your answer should include the type of bonding, the elements contained, and the size and shape of the molecule. write a short paragraph.
Answers: 3


Chemistry, 23.06.2019 18:50, nourmaali
Why are very high temperatures and pressures required for fusion to occur? to generate the neutrons that are needed to break the nuclei o to overcome the repulsion between the protons in the nuclei that join to maintain the proper conditions to keep the chain reaction going to keep the uranium fuel separate from the control rods
Answers: 1

Chemistry, 23.06.2019 19:40, montamonta0204
Which of the following fossils are trace fossils?
Answers: 1
Do you know the correct answer?
Draw the Lewis structure of SF2, showing all lone pairs. Identify the molecular geometry of SF2.
Questions in other subjects:


History, 12.02.2021 22:50



Chemistry, 12.02.2021 22:50

Mathematics, 12.02.2021 22:50

History, 12.02.2021 22:50