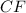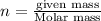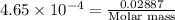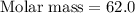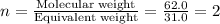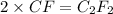Chemistry, 10.03.2020 00:29, jjimenez0276
A 0.02887 g sample of gas occupies 10.0 mL at 288.0 K and 1.10 atm. Upon further analysis, the compound is found to be 38.734 % C and 61.266 % F . What is the molecular formula of the compound?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:30, fbillinton
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 04:40, deedee363
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 06:30, AleciaCassidy
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1
Do you know the correct answer?
A 0.02887 g sample of gas occupies 10.0 mL at 288.0 K and 1.10 atm. Upon further analysis, the compo...
Questions in other subjects:

History, 05.12.2020 20:00


Mathematics, 05.12.2020 20:00



Mathematics, 05.12.2020 20:10

Biology, 05.12.2020 20:10



History, 05.12.2020 20:10