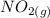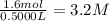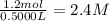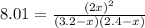Chemistry, 10.03.2020 00:27, TabbyKun00
Suppose a 500.mL flask is filled with 1.6mol of NO3 and 1.2mol of NO2 . The following reaction becomes possible: +NO3gNOg 2NO2g The equilibrium constant K for this reaction is 8.01 at the temperature of the flask. Calculate the equilibrium molarity of NO3 . Round your answer to two decimal places.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:30, lovebaeforlife351
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins. co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 23.06.2019 00:30, mariaramirez110379
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1

Chemistry, 23.06.2019 05:40, girlchamp654
The independent variable in an experiment will be the variable that you o a) change ob) hold constant ng c) observe for changes
Answers: 2
Do you know the correct answer?
Suppose a 500.mL flask is filled with 1.6mol of NO3 and 1.2mol of NO2 . The following reaction becom...
Questions in other subjects:


Business, 30.11.2021 02:00

Mathematics, 30.11.2021 02:00

English, 30.11.2021 02:00



Mathematics, 30.11.2021 02:00




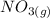 is 1.60 M
is 1.60 M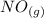 ⇄2
⇄2