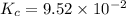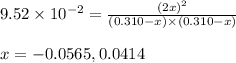He equilibrium constant, Kc, for the following reaction is 9.52×10-2 at 350 K:
CH4(g) + CCl4(g)→ 2CH2Cl2(g)
Calculate the equilibrium concentrations of reactants and product when 0.310 moles of CH4 and 0.310 moles of CCl4 are introduced into a 1.00 L vessel at 350 K.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, YoEsMyles3115
0.2348 grams of pbcl2 used to form 44.0 ml of solution.
Answers: 1

Chemistry, 22.06.2019 17:00, calmicaela12s
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 22.06.2019 19:30, simihehe
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 22.06.2019 22:30, angelagonzalesownus1
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1
Do you know the correct answer?
He equilibrium constant, Kc, for the following reaction is 9.52×10-2 at 350 K:
CH4(g) +...
CH4(g) +...
Questions in other subjects:

Mathematics, 13.04.2021 02:30







Mathematics, 13.04.2021 02:30

Mathematics, 13.04.2021 02:30


 are 0.2686 M, 0.2686 M and 0.0828 M respectively.
are 0.2686 M, 0.2686 M and 0.0828 M respectively.
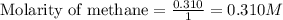
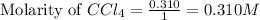
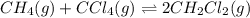
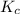 for above equation follows:
for above equation follows:![K_c=\frac{[CH_2Cl_2]^2}{[CH_4][CCl_4]}](/tpl/images/0539/2756/bf52a.png)