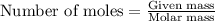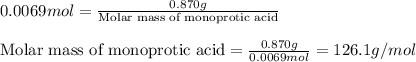A 0.870 g sample of a monoprotic acid is dissolved in water and titrated with 0.300 M KOH.
Wha...

Chemistry, 10.03.2020 00:50, kylediedrich1343
A 0.870 g sample of a monoprotic acid is dissolved in water and titrated with 0.300 M KOH.
What is the molar mass of the acid if 23.0 mL of the KOH solution is required to neutralize the sample?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:30, annanikherrera
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3


Chemistry, 23.06.2019 10:00, jdmXdude3140
How to draw a diagram to represent a calcium metal lattice?
Answers: 3
Do you know the correct answer?
Questions in other subjects:




Mathematics, 28.07.2019 04:34


Mathematics, 28.07.2019 04:34

Physics, 28.07.2019 04:34

Physics, 28.07.2019 04:34

History, 28.07.2019 04:34


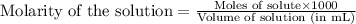

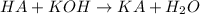
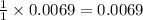 moles of HA
moles of HA