
Enter your answer in the provided box. The equilibrium constant Kc for the equation2H2(g) + CO(g) ⇌ CH3OH(g)is 35 at a certain temperature. If there are 3.21 ×10−2 moles of H2 and 4.87 ×10−3 moles of CH3OH at equilibrium in a 3.63−L flask, what is the concentration of CO?

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:30, americanbellabeauty
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 12:30, AlexRavenwood127
What metric units would you use to measure the thickness of a key
Answers: 3
Do you know the correct answer?
Enter your answer in the provided box. The equilibrium constant Kc for the equation2H2(g) + CO(g) ⇌...
Questions in other subjects:


Mathematics, 05.04.2021 07:50

Mathematics, 05.04.2021 07:50





Mathematics, 05.04.2021 07:50


Arts, 05.04.2021 07:50


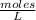
![\frac{[C]^{c}*[D]^{d} }{[A]^{a} *[B]^{b} }](/tpl/images/0539/4311/cbd8b.png)
![Kc=\frac{[CH_{3}OH] }{[H_{2} ]^{2}*[CO] }](/tpl/images/0539/4311/d9c27.png)
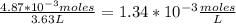 [H₂]=
[H₂]=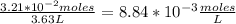 [CO]=?
[CO]=?![35=\frac{1.34*10^{-3} }{(8.84*10^{-3} )^{2} *[CO]}](/tpl/images/0539/4311/9e3f4.png)
![[CO]= \frac{1.34*10^{-3} }{(8.84*10^{-3} )^{2} *35}](/tpl/images/0539/4311/94e86.png)




