

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:30, dyanaycooper13
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 18:30, sarahbug56
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2

Chemistry, 22.06.2019 20:50, iluminatioffial9699
One nanometer is equal to how many meters?
Answers: 2
Do you know the correct answer?
Given the two reactions PbCl2(aq)⇌Pb2+(aq)+2Cl−(aq), K3 = 1.84×10−10, and AgCl(aq)⇌Ag+(aq)+Cl−(aq),...
Questions in other subjects:

Mathematics, 22.08.2019 18:30

Mathematics, 22.08.2019 18:30





Mathematics, 22.08.2019 18:30

Mathematics, 22.08.2019 18:30


Social Studies, 22.08.2019 18:30



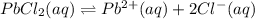 ;
; 
 ;
; 
 ;
; 







