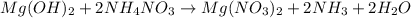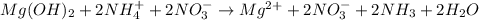Chemistry, 09.03.2020 23:56, SushiMagic
When ammonium nitrate is added to a suspension of magnesium hydroxide in water, the Mg(OH)2 dissolves. Write a net ionic equation to show how this occurs. Do not include physical states and use the smallest possible integer coefficients.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, cutebab4786
Consider the point on the plot where 10.0 g of naoh have been added. what amount of naoh, in moles, has been added? 0.308 mol fecl3 initially present
Answers: 1

Chemistry, 22.06.2019 00:30, TMeansStupidity
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1


Chemistry, 22.06.2019 18:30, kate3887
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1
Do you know the correct answer?
When ammonium nitrate is added to a suspension of magnesium hydroxide in water, the Mg(OH)2 dissolve...
Questions in other subjects:

Mathematics, 29.06.2020 18:01


Mathematics, 29.06.2020 18:01

Mathematics, 29.06.2020 18:01

History, 29.06.2020 18:01



Chemistry, 29.06.2020 18:01


English, 29.06.2020 18:01


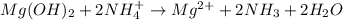
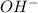 in
in 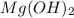 reacts with
reacts with 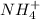 to form
to form 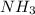 and
and 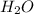 .
.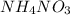 is added to suspension of
is added to suspension of