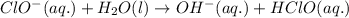Chemistry, 10.03.2020 00:13, bercishicicorbin
Once the ionic solid has dissolved, the anion that is formed is able to react as a base, with water as the acid. Write the net acid-base reaction that occurs when dissolved NaClO reacts with water. (Use the lowest possible coefficients. Omit states-of-matter in your answer.)

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:10, roserose3098
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 15:00, kamkam5791
Is powdered sports drink ionic or covalent ? 10pts !
Answers: 1

Chemistry, 22.06.2019 21:00, cxttiemsp021
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1
Do you know the correct answer?
Once the ionic solid has dissolved, the anion that is formed is able to react as a base, with water...
Questions in other subjects:


Mathematics, 05.10.2020 15:01


Mathematics, 05.10.2020 15:01

English, 05.10.2020 15:01




History, 05.10.2020 15:01


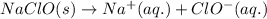
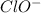 reacts with water to form conjugate acid.
reacts with water to form conjugate acid.