Problem Page Question An analytical chemist weighs out of an unknown monoprotic acid into a volumetric flask and dilutes to the mark with distilled water. He then titrated this solution with solution. When the titration reaches the equivalence point, the chemist finds he has added of solution. Calculate the molar mass of the unknown acid. Round your answer to significant digits.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:30, ansuaprajita1506
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 23:00, Mw3spartan17
What extra step distinguishes fermentation from glycolysis
Answers: 1

Chemistry, 23.06.2019 07:00, Bassoonist
How does science use models to gain a better understanding of concepts?
Answers: 1

Chemistry, 23.06.2019 09:30, kleathers97
Hey, could someone me answer this? much appreciated!
Answers: 1
Do you know the correct answer?
Problem Page Question An analytical chemist weighs out of an unknown monoprotic acid into a volumetr...
Questions in other subjects:

English, 06.07.2019 12:00

Mathematics, 06.07.2019 12:00


Mathematics, 06.07.2019 12:00

Biology, 06.07.2019 12:00

Social Studies, 06.07.2019 12:00



Biology, 06.07.2019 12:00


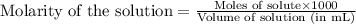
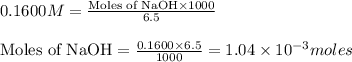

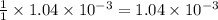 moles of HA
moles of HA