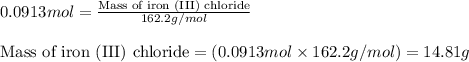Chemistry, 09.03.2020 19:35, demigod0701
When iron(III) oxide reacts with hydrochloric acid, iron(III) chloride and water are formed. How many grams of iron(III) chloride are formed from 10.0 g of iron(III) oxide and 10.0 g of hydrochloric acid

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, 20alondra04
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1

Chemistry, 22.06.2019 06:40, CylieTbh
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3


Chemistry, 23.06.2019 08:00, kathrynpuppies201716
At 35.0°c and 3.00 atm pressure, a gas has a volume of 1.40 l. what pressure does the gas have at 0.00°c and a volume of 0.950 l? which equation should you use? p2= p1v1t2/t1v2what is the pressure of the gas? 3.92 atm these are the answers
Answers: 1
Do you know the correct answer?
When iron(III) oxide reacts with hydrochloric acid, iron(III) chloride and water are formed. How man...
Questions in other subjects:



History, 03.03.2021 01:00

English, 03.03.2021 01:00


Mathematics, 03.03.2021 01:00

Mathematics, 03.03.2021 01:00




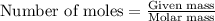 .....(1)
.....(1)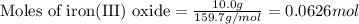
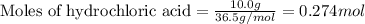
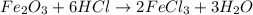
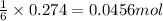 of iron (III) oxide
of iron (III) oxide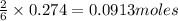 of iron (III) chloride
of iron (III) chloride