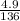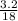Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:00, valdezlizbeth6652
Why is carbon ideal for making different compounds?
Answers: 2

Chemistry, 22.06.2019 20:00, bbyitskeke7160
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3

Chemistry, 22.06.2019 22:30, darceline1574
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
Do you know the correct answer?
8.1grams of xso4.7H2O when heated at 90°c for 15minutes gave 4.9grams when heated further at 120°c....
Questions in other subjects:

Mathematics, 24.08.2019 06:00

Physics, 24.08.2019 06:00

History, 24.08.2019 06:00

Social Studies, 24.08.2019 06:00





Physics, 24.08.2019 06:00