
Chemistry, 08.03.2020 21:11, genyjoannerubiera
C2 H4+ 3O2= 2CO2+2H2O how many moles of methane will be burnt to produce 0.1 of water

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, andybiersack154
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 02:40, hardwick744
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2

Chemistry, 22.06.2019 04:30, akeemedwards12
Both josef loschmidt and amedeo avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. neither scientist discovered “avogadro’s number” in the form we use it today (6.02 x 10 23). still, there’s a controversy over the name. research the contributions from these two scientists and read about how avogadro’s number got its name. briefly state what you think this number should be called, providing key details of each scientist’s contributions to this concept and a solid rationale for your case in naming the number.
Answers: 2

Chemistry, 22.06.2019 05:30, medlinalex
Compare and contrast physical changes with chemical changes.
Answers: 1
Do you know the correct answer?
C2 H4+ 3O2= 2CO2+2H2O how many moles of methane will be burnt to produce 0.1 of water...
Questions in other subjects:




Chemistry, 09.11.2020 16:00

English, 09.11.2020 16:00

English, 09.11.2020 16:00



Mathematics, 09.11.2020 16:00

English, 09.11.2020 16:00


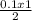 = 0.05 mole of C₂H₄
= 0.05 mole of C₂H₄



