
A sample of an ideal gas at 1.00 atm and a volume of 1.32 L was placed in a weighted balloon and dropped into the ocean. As the sample descended, the water pressure compressed the balloon and reduced its volume. When the pressure had increased to 90.0 atm , what was the volume of the sample

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 00:00, alisonsolis155
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3


Chemistry, 23.06.2019 18:30, RealSavage4Life
Select the correct answer. which statement best explains the octet rule? a. atoms gain, lose, or share electrons to achieve a full valence shell. b. compounds always consist of eight electrons. c. molecules always consist of eight atoms. d. all atoms require eight electrons to form bonds.
Answers: 2
Do you know the correct answer?
A sample of an ideal gas at 1.00 atm and a volume of 1.32 L was placed in a weighted balloon and dro...
Questions in other subjects:




Mathematics, 23.09.2019 05:30



Social Studies, 23.09.2019 05:30



History, 23.09.2019 05:30


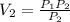 that is V₂ =
that is V₂ = 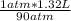 =
= 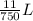 or 0.01467L
or 0.01467L



