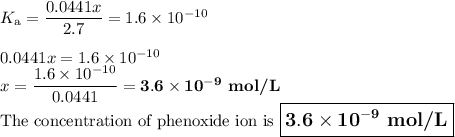Chemistry, 07.03.2020 06:00, andrewmena05
Calculate the pH of a solution that contains 2.7 M HF and 2.7 M HOC6H5. Also, calculate the concentration of OC6H5- in this solution at equilibrium. Ka(HF) = 7.2×10-4; Ka(HOC6H5) = 1.6×10-10. pH = [OC6H5-] = M

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 19:30, 2020sanchezyiczela
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
Do you know the correct answer?
Calculate the pH of a solution that contains 2.7 M HF and 2.7 M HOC6H5. Also, calculate the concentr...
Questions in other subjects:

Mathematics, 12.09.2020 01:01

Mathematics, 12.09.2020 01:01

Social Studies, 12.09.2020 01:01

Mathematics, 12.09.2020 01:01

World Languages, 12.09.2020 01:01

Mathematics, 12.09.2020 01:01

Mathematics, 12.09.2020 01:01

Mathematics, 12.09.2020 01:01

History, 12.09.2020 01:01

Mathematics, 12.09.2020 01:01


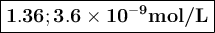
![K_{\text{a}} = \dfrac{\text{[H}_{3}\text{O}^{+}] \text{F}^{-}]} {\text{[HF]}} = 7.2 \times 10^{-4}\\\\\dfrac{x^{2}}{2.7 - x} = 7.2 \times 10^{-4}\\\\\text{Check for negligibility of }x\\\\\dfrac{2.7}{7.2 \times 10^{-4}} = 4000 400\\\\\therefore x \ll 2.7\\\dfrac{x^{2}}{2.7} = 7.2 \times 10^{-4}\\\\x^{2} = 2.7 \times 7.2 \times 10^{-4} = 1.94 \times 10^{-3}\\x = 0.0441\\\text{[H$_{3}$O$^{+}$]}= \text{x mol$\cdot$L$^{-1}$} = \text{0.0441 mol$\cdot$L$^{-1}$}](/tpl/images/0538/0586/c13f6.png)
![\text{pH} = -\log{\rm[H_{3}O^{+}]} = -\log{0.0441} = \large \boxed{\mathbf{1.36}}](/tpl/images/0538/0586/4731b.png)