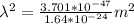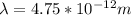A particle confined to rotate on a sphere is a very good approximation for the rotations ofdiatomic molecules. Using the equation for the energy of a particle rotating on a sphere, compute the equilibrium bond length of H137I, given that the rotational energy in the`= 5state is 8.952×10−21J. HINT: You will need to use the reduced mass.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, ayaanwaseem
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 09:20, UsedForSchool2018
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 13:30, makenziehook8
Which is true of a liquid? it has a definite volume but not a definite mass. it has a definite mass but not a definite volume. it has a definite volume but not a definite shape. it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 21:30, jpimentel2021
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2
Do you know the correct answer?
A particle confined to rotate on a sphere is a very good approximation for the rotations ofdiatomic...
Questions in other subjects:

English, 13.11.2020 01:00

Spanish, 13.11.2020 01:00


Mathematics, 13.11.2020 01:00


Mathematics, 13.11.2020 01:00


History, 13.11.2020 01:00

Biology, 13.11.2020 01:00


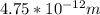
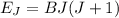
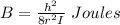
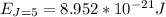
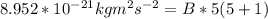
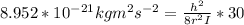
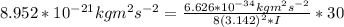
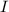 the subject
the subject 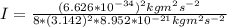
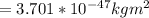
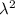
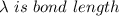
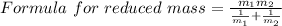
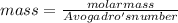
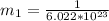
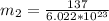

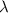 the subject of the formula
the subject of the formula