
Chemistry, 07.03.2020 05:26, juanesmania
A gas of unknown molecular mass was allowed to effuse through a small opening under constant-pressure conditions. It required 105 s for 1.0 L of the gas to effuse. Under identical experimental conditions it required 30 s for 1.0 L of O2 gas to effuse. Calculate the molar mass of the unknown gas.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, emilyproce
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 16:30, Kathryn014
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

Chemistry, 22.06.2019 22:30, SavageKidKobe
Rank the four gases (air, exhaled air, gas produced from from decomposition of h2o2, gas from decomposition of nahco3) in order of increasing concentration of co2
Answers: 1
Do you know the correct answer?
A gas of unknown molecular mass was allowed to effuse through a small opening under constant-pressur...
Questions in other subjects:

History, 26.07.2019 18:30

Mathematics, 26.07.2019 18:30





English, 26.07.2019 18:30


Mathematics, 26.07.2019 18:30

Mathematics, 26.07.2019 18:30


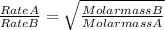
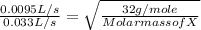 or Molar mass of X =
or Molar mass of X = 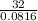 g/mole = 391.99 g/mole
g/mole = 391.99 g/mole



