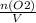Chemistry, 07.03.2020 05:26, kfcnkfnmnfk9513
A sample of pure NO2 is heated to 337?C at which temperature it partially dissociates according to the equation2NO2(g)?2NO(g)+O2(g)At equilibrium the density of the gas mixture is 0.525g/L at 0.745atm . Calculate Kc for the reaction.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, officialgraciela67
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 05:50, vanessa051266
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 10:00, melissa9882
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 18:00, brisacruz013
Which statement best describes the he properties of iconic compounds ?
Answers: 1
Do you know the correct answer?
A sample of pure NO2 is heated to 337?C at which temperature it partially dissociates according to t...
Questions in other subjects:


Physics, 15.12.2019 00:31




Mathematics, 15.12.2019 00:31

Mathematics, 15.12.2019 00:31



Biology, 15.12.2019 00:31


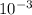
![\frac{[NO]^{2} .[O2]}{[NO2]^{2} }](/tpl/images/0537/9415/78cff.png)
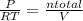
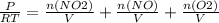
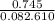 = 0.015 mol/L
= 0.015 mol/L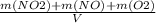
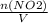 +M(NO)·
+M(NO)·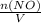 +M(O2)·
+M(O2)·