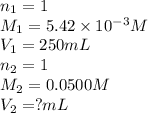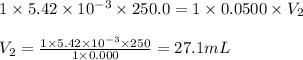Chemistry, 07.03.2020 05:41, alexandria3498
A chemistry student weighs out of acrylic acid into a volumetric flask and dilutes to the mark with distilled water. He plans to titrate the acid with solution. Calculate the volume of solution the student will need to add to reach the equivalence point. Be sure your answer has the correct number of significant digits.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:10, nauticatyson9
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 23.06.2019 10:00, Jennifer16253
What is the mass in grams of 12.26 ml of acetone
Answers: 1

Chemistry, 23.06.2019 10:50, reesespowerade
Achemist reacted 57.50 grams of sodium metal with an excess amount of chlorine gas. the chemical reaction that occurred is shown. na + cl2 → nacl if the percentage yield of the reaction is 86%, what is the actual yield? show your work, including the use of stoichiometric calculations and conversion factors.
Answers: 1
Do you know the correct answer?
A chemistry student weighs out of acrylic acid into a volumetric flask and dilutes to the mark with...
Questions in other subjects:

Mathematics, 20.05.2020 19:59

Biology, 20.05.2020 19:59




Biology, 20.05.2020 19:59

Mathematics, 20.05.2020 19:59

History, 20.05.2020 19:59

Biology, 20.05.2020 19:59

Mathematics, 20.05.2020 20:57


 into a 250. mL volumetric flask and diluted to the mark with distilled water. He plans to titrate the acid with 0.0500 M NaOH solution. Calculate the volume of NaOH solution the student will need to add to reach the equilvalence point. Be sure your answer has the correct number of significant digits.
into a 250. mL volumetric flask and diluted to the mark with distilled water. He plans to titrate the acid with 0.0500 M NaOH solution. Calculate the volume of NaOH solution the student will need to add to reach the equilvalence point. Be sure your answer has the correct number of significant digits.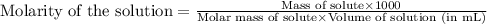
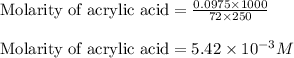
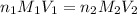
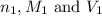 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 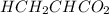
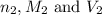 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.