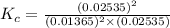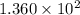Chemistry, 07.03.2020 05:42, jasminebrown72
Before any reaction occurs, the concentrations of A and B in the reaction below are each 0.03900 M . What is the equilibrium constant if the concentration of A at equilibrium is 0.01365 M ? 2A(aq)+B(aq)⇌2C(aq) Round your answer to one decimal place.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, nikejose11
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 08:30, aydenmasonc
Which statement describes james chadwick’s discovery.
Answers: 2

Chemistry, 22.06.2019 14:00, BrandyLeach01
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3

Chemistry, 22.06.2019 20:00, kalcius9698
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
Do you know the correct answer?
Before any reaction occurs, the concentrations of A and B in the reaction below are each 0.03900 M ....
Questions in other subjects:






Mathematics, 05.05.2020 23:12

History, 05.05.2020 23:12




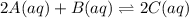
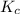 of the given reaction is as follows.
of the given reaction is as follows.![K_{c} = \frac{[C]^{2}}{[A]^{2}[B]}](/tpl/images/0538/0202/8ca5b.png)