
Chemistry, 07.03.2020 05:21, juanitarodriguez
Calculate the entropy change when a. two moles of H2O(g) are cooled irreversible at constant p from 120°C to 100°C. b. one mole of H2O(g) is expanded at constant pressure of 2 bar from an original volume of 20 L to a final volume of 25 L. You can consider the gas to be ideal. c. one hundred grams of H2O(s) at -10°C and 1 bar are heated to H2O(l) at +10°C and 1 bar.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:10, tishfaco5000
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 04:40, marknjenbennetp3j1v1
Listen base your answer to the question on the information below. propane is a fuel that is sold in rigid, pressurized cylinders. most of the propane in a cylinder is liquid, with gas in the space above the liquid level. when propane is released from the cylinder, the propane leaves the cylinder as a gas. propane gas is used as a fuel by mixing it with oxygen in the air and igniting the mixture, as represented by the balanced equation below. c3h8(g) + 5o2(g) → 3co2(g) + 4h2o() + 2219.2 kja small amount of methanethiol, which has a distinct odor, is added to the propane to consumers detect a propane leak. in methanethiol, the odor is caused by the thiol functional group (–sh). methanethiol, ch3sh, has a structure that is very similar to the structure of methanol. what is the correct structural formula for a molecule of methanethiol
Answers: 3


Chemistry, 22.06.2019 07:00, vivianni0727p1y30v
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2
Do you know the correct answer?
Calculate the entropy change when a. two moles of H2O(g) are cooled irreversible at constant p from...
Questions in other subjects:



Mathematics, 26.03.2021 01:00




Social Studies, 26.03.2021 01:00

Mathematics, 26.03.2021 01:00

Mathematics, 26.03.2021 01:00

Mathematics, 26.03.2021 01:00


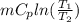 Where
Where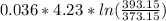 = 0.00795 kJ/(K)
= 0.00795 kJ/(K) 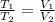 Therefore change in entropy S₂ - S₁ =
Therefore change in entropy S₂ - S₁ =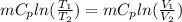
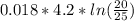 = -0.01686965 kJ/(K) = -16.9 J/K
= -0.01686965 kJ/(K) = -16.9 J/K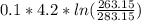 = -0.03078 kJ/K
= -0.03078 kJ/K




