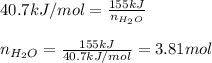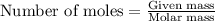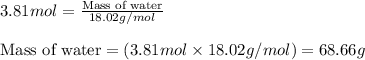Chemistry, 07.03.2020 05:07, moningersavannah
Calculate the mass of water (in g) that can be vaporized at its boiling point with 155 kJ of heat. • ΔHvap = 40.7 kJ/mol (at 100 °C) • 18.02 g H2O = 1 mol H2O

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:30, kevin72937
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 22.06.2019 18:00, ameliaxbowen7
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1
Do you know the correct answer?
Calculate the mass of water (in g) that can be vaporized at its boiling point with 155 kJ of heat. •...
Questions in other subjects:






Health, 05.05.2020 16:28

Computers and Technology, 05.05.2020 16:28


Physics, 05.05.2020 16:28

Mathematics, 05.05.2020 16:28


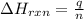
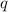 = amount of heat absorbed = 155 kJ
= amount of heat absorbed = 155 kJ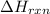 = enthalpy change of the reaction = 40.7 kJ/mol
= enthalpy change of the reaction = 40.7 kJ/mol