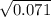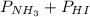Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, AbhiramAkella
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1

Chemistry, 22.06.2019 09:40, gonzaleze18
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2


Chemistry, 22.06.2019 20:00, batoolishak7475
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
Do you know the correct answer?
If Kp For This Reaction Is 0.071 At 100 °C (when The Partial Pressures Are Measured In Atmospheres),...
Questions in other subjects:

Biology, 25.12.2019 06:31


Mathematics, 25.12.2019 06:31




Mathematics, 25.12.2019 06:31

History, 25.12.2019 06:31



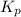 = 0.071 at 100°C
= 0.071 at 100°C![[P_{NH_3}][P_{HI}]](/tpl/images/0537/6634/19fd4.png)
![[x][x]](/tpl/images/0537/6634/ce5d5.png)