

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, timiaparker
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 10:10, dhailyortegacampa131
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 14:10, roserose3098
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 19:40, trodgers0202
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests. which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3
Do you know the correct answer?
Consider the following reaction, equilibrium concentrations, and equilibrium constant at a particula...
Questions in other subjects:



Social Studies, 15.04.2020 18:42

History, 15.04.2020 18:42







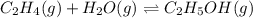
![[C_2H_4]=0.015M](/tpl/images/0537/7510/e5922.png)
![[H_2O]=?](/tpl/images/0537/7510/d3a13.png)
![[C_2H_5OH]=1.69 M](/tpl/images/0537/7510/ae95d.png)
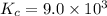
![K_c=\frac{[C_2H_5OH]}{[C_2H_4][H_2O]}](/tpl/images/0537/7510/88e9b.png)
![9.0\times 10^3=\frac{1.69 M}{0.015 M\times [H_2O]}](/tpl/images/0537/7510/9ae4d.png)
![[H_2O]=\frac{1.69 M}{0.015 M\times 9\timers 10^3}=0.0125 M](/tpl/images/0537/7510/e2bbe.png)




