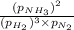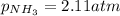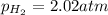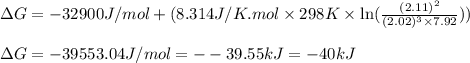Chemistry, 07.03.2020 04:48, Mattisback2285
A chemist fills a reaction vessel with 7.92 atm nitrogen (N2) gas, 2.02 atm hydrogen (H2) gas, and 2.11 atm ammonia (NH3) gas at a temperature of 25.0°C
Under these conditions, calculate the reaction free energy ΔG for the following chemical reaction
N2(8) +3H2 2NH3 (g)
Use the thermodynamic information in the ALEKS Data tab. Round your answer to the nearest kilojoule.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, zayam1626
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 19:40, trodgers0202
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests. which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3

Chemistry, 23.06.2019 01:50, UncleVictor5188
Ablock of aluminum is dropped into a graduated cylinder with an initial volume of water at 75ml and the volumes rises to 90ml. if the block has a mass of 40.5 g what is its density ?
Answers: 1
Do you know the correct answer?
A chemist fills a reaction vessel with 7.92 atm nitrogen (N2) gas, 2.02 atm hydrogen (H2) gas, and 2...
Questions in other subjects:


Mathematics, 21.05.2021 20:30


Geography, 21.05.2021 20:30



Advanced Placement (AP), 21.05.2021 20:30

Biology, 21.05.2021 20:30

Mathematics, 21.05.2021 20:30


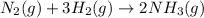
![\Delta G^o_{rxn}=[(2\times \Delta G^o_f_{(NH_3(g))})]-[(1\times \Delta G^o_f_{(N_2(g))})+(3\times \Delta G^o_f_{(H_2(g))})]](/tpl/images/0537/7676/6e73c.png)

![\Delta G^o_{rxn}=[(2\times (-16.45))]-[(1\times (0))+(3\times (0))]\\\\\Delta G^o_{rxn}=-32.9kJ/mol](/tpl/images/0537/7676/8bdb6.png)
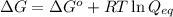
 = free energy of the reaction
= free energy of the reaction = standard Gibbs free energy = -32.9 kJ/mol = -32900 J/mol (Conversion factor: 1 kJ = 1000 J)
= standard Gibbs free energy = -32.9 kJ/mol = -32900 J/mol (Conversion factor: 1 kJ = 1000 J)![25^oC=[273+25]K=298K](/tpl/images/0537/7676/0e82f.png)
 = Ratio of concentration of products and reactants at any time =
= Ratio of concentration of products and reactants at any time =