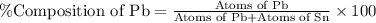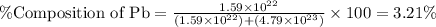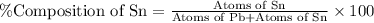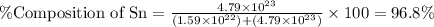Chemistry, 07.03.2020 04:57, fatherbamboo
What is the composition, in atom percent, of an alloy that consists of a) 5.5 wt% Pb and b) 94.5 wt% of Sn? Assume that the atomic weight for lead and tin are 207.2 and 118.71 g/mol, respectively.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 00:30, evelynalper08
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2

Chemistry, 23.06.2019 02:30, micahwilkerson9495
Which words or phrases identify layers of groundwater? check all that apply. water table kettle lake saturation zone underground lake sinkhole will give brainiest, answer quickly.
Answers: 1

Chemistry, 23.06.2019 05:30, Dallas3506
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1
Do you know the correct answer?
What is the composition, in atom percent, of an alloy that consists of a) 5.5 wt% Pb and b) 94.5 wt%...
Questions in other subjects:


Mathematics, 09.10.2019 20:30


History, 09.10.2019 20:30

Mathematics, 09.10.2019 20:30


English, 09.10.2019 20:30


English, 09.10.2019 20:30

Mathematics, 09.10.2019 20:30


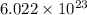 atoms
atoms atoms
atoms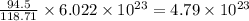 atoms
atoms