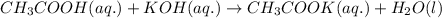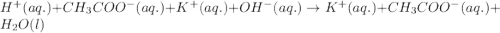Chemistry, 07.03.2020 04:57, BlehBlehBlehBleh
Equal volumes of 0.25 M acetic acid and 0.25 M potassium hydroxide are combined. Write the net ionic equation for the reaction and identify the aqueous species that have the highest concentrations at equilibrium. Justify your answer.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, kaylaamberd
Write the complete balanced equation for the reaction between lead (iv) oxide (pbo2) and water (h2o).
Answers: 1


Chemistry, 22.06.2019 15:30, lizzyhearts
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 19:00, nayashuntel
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1
Do you know the correct answer?
Equal volumes of 0.25 M acetic acid and 0.25 M potassium hydroxide are combined. Write the net ionic...
Questions in other subjects:


Chemistry, 05.07.2021 02:40

Law, 05.07.2021 02:40


History, 05.07.2021 02:40

Mathematics, 05.07.2021 02:40


Mathematics, 05.07.2021 02:40


Mathematics, 05.07.2021 02:40


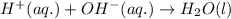 and all the ions have equal concentrations at equilibrium
and all the ions have equal concentrations at equilibrium