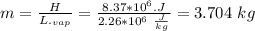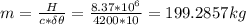Chemistry, 07.03.2020 03:56, damiangibson2
Assume that a daily diet of 2000 calories (i. e. 8.37 x 106 J) is converted completely to body heat.
a) How many g of water (as sweat) would need to evaporate to cool that person off?
b) If instead of evaporating water, the heat was used to raise the temperature of some water from 25.0 °C to 35.0 °C, how much water could be heated?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:10, rightstrong9827
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3

Chemistry, 22.06.2019 12:30, Svetakotok
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2


Chemistry, 23.06.2019 00:30, mathwiznot45
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1
Do you know the correct answer?
Assume that a daily diet of 2000 calories (i. e. 8.37 x 106 J) is converted completely to body heat....
Questions in other subjects:



History, 10.03.2020 16:07


Mathematics, 10.03.2020 16:07


Mathematics, 10.03.2020 16:07

English, 10.03.2020 16:07


English, 10.03.2020 16:07