
Chemistry, 07.03.2020 04:14, mckleinrivero
The rate constant for a certain reaction is kkk = 3.50×10−3 s−1s−1 . If the initial reactant concentration was 0.450 MM, what will the concentration be after 19.0 minutes?
A zero-order reaction has a constant rate of 3.50×10−4 M/sM/s. If after 65.0 seconds the concentration has dropped to 3.50×10−2 MM, what was the initial concentration?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, heavyhearttim
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2



Chemistry, 22.06.2019 17:00, smelcher3900
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2
Do you know the correct answer?
The rate constant for a certain reaction is kkk = 3.50×10−3 s−1s−1 . If the initial reactant concent...
Questions in other subjects:


Mathematics, 24.03.2020 20:04

History, 24.03.2020 20:04


Biology, 24.03.2020 20:05


Mathematics, 24.03.2020 20:05



Mathematics, 24.03.2020 20:05


![Rate = k[A][B]](/tpl/images/0537/5969/ab111.png) . As the data are given in the question, the rate constant is
. As the data are given in the question, the rate constant is 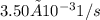 , the initial concentration
, the initial concentration![[A]_o = 0.450 M](/tpl/images/0537/5969/1d14c.png) and time is 19 minutes that is the 1140s.
and time is 19 minutes that is the 1140s.
![ln[A] = ln[A]_o - kt\\ln[A] = ln (0.450) - (3.50*10^-^3)1140\\ln[A] = -0.799 - 3.99\\ln[A] = -4.789\\[A] = 0.0083M](/tpl/images/0537/5969/91bfd.png)
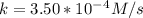 Time = 65 s
Final Concentration [A] =
Time = 65 s
Final Concentration [A] = 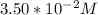
![[A] = [A]_o - kt[A]_o = [A] + kt\\[A]o = 3.50*10^{-2} + (3.50*10^{-4}) * 65\\[A]_o = 3.50*10^{-2} + 0.02275\\[A]_o = 0.05775 M](/tpl/images/0537/5969/3e5cb.png)




