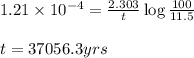Chemistry, 07.03.2020 03:32, jodygoodwin40
Carbon-14 has a half-life of 5720 years and this is a first-order reaction. If a piece of wood has converted 88.5% of the carbon-14, then how old is it?

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:30, Schoolworkspace453
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2

Chemistry, 22.06.2019 23:30, sanociahnoel
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
Do you know the correct answer?
Carbon-14 has a half-life of 5720 years and this is a first-order reaction. If a piece of wood has c...
Questions in other subjects:



Mathematics, 24.09.2021 14:00

Mathematics, 24.09.2021 14:00

Physics, 24.09.2021 14:00





Geography, 24.09.2021 14:00


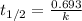
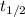 = half life of the reaction = 5720 years
= half life of the reaction = 5720 years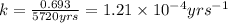
![k=\frac{2.303}{t}\log\frac{[A_o]}{[A]}](/tpl/images/0537/4096/f1041.png)
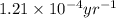
![[A_o]](/tpl/images/0537/4096/dc622.png) = initial amount of the sample = 100 grams
= initial amount of the sample = 100 grams