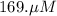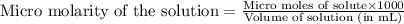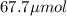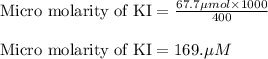Chemistry, 07.03.2020 03:40, ayoismeisalex
A chemist prepares a solution of potassium iodide (KI) by measuring out 67.7. mu mol of potassium iodide into a 400. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mmo1L of the chemist's potassium iodide solution. Round your answer to 3 significant digits.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, kristineford198
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3


Chemistry, 22.06.2019 04:00, armahoney8566
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 09:40, keiracoles
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1
Do you know the correct answer?
A chemist prepares a solution of potassium iodide (KI) by measuring out 67.7. mu mol of potassium io...
Questions in other subjects:

English, 04.01.2020 01:31

History, 04.01.2020 01:31

Business, 04.01.2020 01:31

Mathematics, 04.01.2020 01:31

History, 04.01.2020 01:31



History, 04.01.2020 01:31