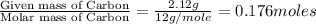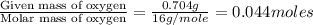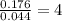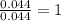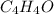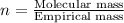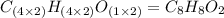Chemistry, 07.03.2020 03:46, dakotaadkins20
3.00 g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 136. g/mol, is burned completely in excess oxygen, and the mass of the products carefully measured product mass water .59 g Use this information to find the molecular formula of X.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:10, jasondesatnick
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 08:30, kkelley9223
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 14:00, BrandyLeach01
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3
Do you know the correct answer?
3.00 g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have...
Questions in other subjects:

Mathematics, 17.12.2020 09:50


Mathematics, 17.12.2020 09:50


Computers and Technology, 17.12.2020 09:50

Mathematics, 17.12.2020 09:50

Mathematics, 17.12.2020 09:50

Mathematics, 17.12.2020 09:50

German, 17.12.2020 09:50

Mathematics, 17.12.2020 09:50



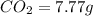
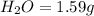
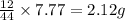 of carbon will be contained.
of carbon will be contained.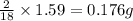 of hydrogen will be contained.
of hydrogen will be contained.