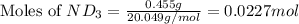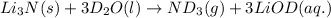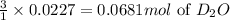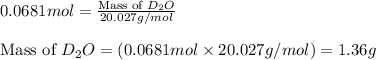Lithium nitride reacts with water to produce ammonia and lithium hydroxide according to the equation Li 3 N ( s ) + 3 H 2 O ( l ) ⟶ NH 3 ( g ) + 3 LiOH ( aq ) Heavy water is water with the isotope deuterium in place of ordinary hydrogen, and its formula is D 2 O . The same reaction can be used to produce heavy ammonia, ND 3 ( g ) , according to the equation Li 3 N ( s ) + 3 D 2 O ( l ) ⟶ ND 3 ( g ) + 3 LiOD ( aq ) Calculate how many grams of heavy water are required to produce 455.0 mg ND 3 ( g ) . The mass of deuterium, D , is 2.014 g/mol.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, daryondaniels28
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2


Chemistry, 22.06.2019 06:30, dimondqueen511
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 12:30, meghan2529
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1
Do you know the correct answer?
Lithium nitride reacts with water to produce ammonia and lithium hydroxide according to the equation...
Questions in other subjects:



Mathematics, 16.04.2021 01:00

Social Studies, 16.04.2021 01:00


Mathematics, 16.04.2021 01:00

Chemistry, 16.04.2021 01:00


Mathematics, 16.04.2021 01:00


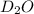 needed is 1.36 grams
needed is 1.36 grams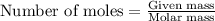 .....(1)
.....(1)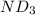 :
: