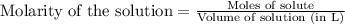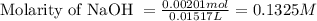Chemistry, 07.03.2020 02:37, loopysoop5035
A 0.411 g sample of potassium hydrogen phthalate, KHC8H4O4 (MM = 204.44 g/mol) is dissolved in 50 mL of deionized water in a 125-Erlenmeyer flask. The sample is titrated to the phenolphthalein endpoint with 15.17 mL of a sodium hydroxide solution. What is the molar concentration of the NaOH solution?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, kylieweeks052704
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2


Chemistry, 22.06.2019 20:00, emilyswinge4421
Listenbase your answer to the question on the information below. nuclear radiation is harmful to living cells, particularly to fast-growing cells, such as cancer cells and blood cells. an external beam of the radiation emitted from a radioisotope can be directed on a small area of a person to destroy cancer cells within the body. cobalt-60 is an artificially produced radioisotope that emits gamma rays and beta particles. one hospital keeps a 100.0-gram sample of cobalt-60 in an appropriate, secure storage container for future cancer treatment. which choice represents the correct product for the beta decay of the co-60? fe-60ni-60fe-61ni-61
Answers: 2

Chemistry, 23.06.2019 03:30, uniqueray33
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1
Do you know the correct answer?
A 0.411 g sample of potassium hydrogen phthalate, KHC8H4O4 (MM = 204.44 g/mol) is dissolved in 50 mL...
Questions in other subjects:




Biology, 10.08.2019 00:30

Mathematics, 10.08.2019 00:30


Mathematics, 10.08.2019 00:30


Biology, 10.08.2019 00:30

Biology, 10.08.2019 00:30


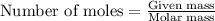
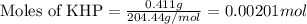
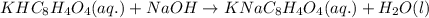
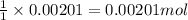 of NaOH.
of NaOH.