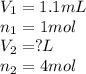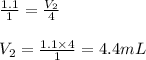Chemistry, 07.03.2020 02:29, homework1911
Methane gas and chlorine gas react to form hydrogen chloride gas and carbon tetrachloride gas. What volume of hydrogen chloride would be produced by this reaction if of methane were consumed? Also, be sure your answer has a unit symbol, and

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:00, meowmeowcow
Find the mass, in grams, of 5.00*10^23 molecules of f2
Answers: 3

Chemistry, 22.06.2019 20:20, catchonyet
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
Do you know the correct answer?
Methane gas and chlorine gas react to form hydrogen chloride gas and carbon tetrachloride gas. What...
Questions in other subjects:



Mathematics, 02.09.2021 09:10


Biology, 02.09.2021 09:10



English, 02.09.2021 09:10

Mathematics, 02.09.2021 09:10

Mathematics, 02.09.2021 09:10


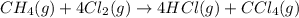
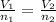
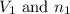 are the volume and number of moles of methane gas
are the volume and number of moles of methane gas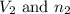 are the volume and number of moles of hydrogen chloride
are the volume and number of moles of hydrogen chloride