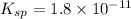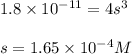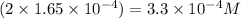Chemistry, 07.03.2020 02:24, avisconti571
A 350 mL saturated solution of magnesium hydroxide, Mg(OH)2 is prepared at 25 degrees Celsius. The solubility product constant, Ksp, for Mg(OH)2 is 1.8 x 10^-11 at 25 degrees Celsius.
1) Find the molar solubility of Mg(OH)2 at 25 degrees Celsius?
2) What is the concentration (mol/L) of hydroxide ion, OH-, in the saturated solution at 25 degrees Celsius?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, jabper5522
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 05:50, mrylenastewart
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 06:30, yolo123321
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 23.06.2019 01:00, shartiarahoward
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3
Do you know the correct answer?
A 350 mL saturated solution of magnesium hydroxide, Mg(OH)2 is prepared at 25 degrees Celsius. The s...
Questions in other subjects:



History, 25.01.2021 23:30

Social Studies, 25.01.2021 23:30






Mathematics, 25.01.2021 23:30


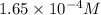
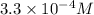
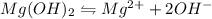
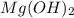 will be:
will be:![K_{sp}=[Mg^{2+}][OH^-]^2\\\\K_{sp}=s\times (2s)^2=4s^3](/tpl/images/0537/0995/7d1c0.png)