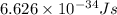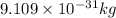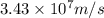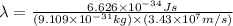Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, queenkimm26
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 10:30, jahmira96
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Do you know the correct answer?
What is the wavelength of an electron with a mass of 9.109×10−31 kg and a velocity of 3.43×107 ms? U...
Questions in other subjects:

Mathematics, 30.08.2020 02:01



Mathematics, 30.08.2020 02:01



Mathematics, 30.08.2020 02:01

Social Studies, 30.08.2020 02:01


Mathematics, 30.08.2020 02:01


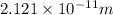
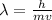
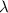 = De-Broglie's wavelength = ?
= De-Broglie's wavelength = ?