Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 23:30, sweaversw9602
What are the similarities between compounds and mixtures?
Answers: 3

Chemistry, 23.06.2019 01:30, oliviacolaizzi
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1

Chemistry, 23.06.2019 08:00, oopsorry
Determine the number of moles of air present in 1.35 l at 750 torr and 17.0°c. which equation should you use? n=pv/rt what is the number of moles present? ⇒ 0.056 mol a sample of n2 gas occupying 800.0 ml at 20.0°c is chilled on ice to 0.00°c. if the pressure also drops from 1.50 atm to 1.20 atm, what is the final volume of the gas? which equation should you use? v2= p1v1t2/p2t1 what is the final volume of the gas? ⇒ 932 ml these are the answers
Answers: 1
Do you know the correct answer?
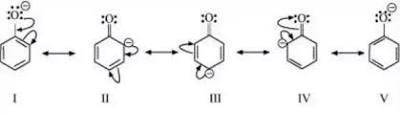
Draw the resonance structures for the conjugate base of Phenol (C6H6O). In one sentence, explain why...
Questions in other subjects:


Mathematics, 23.04.2021 21:30




Mathematics, 23.04.2021 21:30

German, 23.04.2021 21:30


Mathematics, 23.04.2021 21:30

Biology, 23.04.2021 21:30