

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 23:00, jolainjoseph01998
What element has similar physical and chemical properties as boron.
Answers: 1


Chemistry, 23.06.2019 05:00, sCoTtYbOy5329
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 1
Do you know the correct answer?
A weak acid is titrated with 0.1236 M NaOH. From the titration curve you determine that the equivale...
Questions in other subjects:


History, 09.12.2020 04:30

Mathematics, 09.12.2020 04:30

Health, 09.12.2020 04:30


Computers and Technology, 09.12.2020 04:30

Mathematics, 09.12.2020 04:30




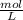 × 12.43 mL
× 12.43 mL



