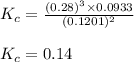Problem PageQuestion Ammonia will decompose into nitrogen and hydrogen at high temperature. An industrial chemist studying this reaction fills a 75.0L tank with 23. mol of ammonia gas at 48.°C. He then raises the temperature, and when the mixture has come to equilibrium measures the amount of hydrogen gas to be 21. mol. Calculate the concentration equilibrium constant for the decomposition of ammonia at the final temperature of the mixture. Round your answer to 2 significant digits.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, luludawn2455
Dying the folding patterns of protein molecules can microbiologists better understand cellular processes as well as some diseases, such as alzheimer’s, that are caused by proteins that have misfolded. the folding of these complicated molecules can be simulated on computers, but it takes a lot of processor power and time for even expensive supercomputers to do this. a group of researchers at stanford university developed software that can be used to distribute the processing of data to anyone who is willing to donate time on their idle personal computers. as a result, the researchers have been able to achieve protein-folding simulations that are far better than those other computing methods have done. which statement best describes the work of these researchers? the work is not scientific because the data are not processed in one location. the work is not scientific because the simulations are not reproducible. the researchers applied creativity to solve a problem in running an experiment. the researchers used only well-established scientific techniques.
Answers: 3



Chemistry, 22.06.2019 13:30, hdhtvthjr
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1
Do you know the correct answer?
Problem PageQuestion Ammonia will decompose into nitrogen and hydrogen at high temperature. An indus...
Questions in other subjects:


History, 26.01.2021 05:40









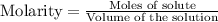
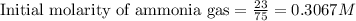
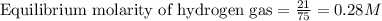
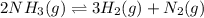
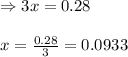
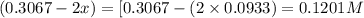
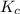 for above equation follows:
for above equation follows:![K_c=\frac{[H_2]^3[N_2]}{[NH_3]^2}](/tpl/images/0536/9367/5cab9.png)