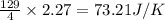Chemistry, 07.03.2020 00:46, flowerrbabie
Consider the reaction 4HCl(g) + O2(g)2H2O(g) + 2Cl2(g) Using standard thermodynamic data at 298K, calculate the entropy change for the surroundings when 2.27 moles of HCl(g) react at standard conditions. Ssurroundings = J/K

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:30, andrejr0330jr
What is the molar mass of potassium nitrate, kno3
Answers: 1

Chemistry, 22.06.2019 14:10, roserose3098
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 14:30, lorrainelopez
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1
Do you know the correct answer?
Consider the reaction 4HCl(g) + O2(g)2H2O(g) + 2Cl2(g) Using standard thermodynamic data at 298K, ca...
Questions in other subjects:

Mathematics, 22.06.2019 06:30




History, 22.06.2019 06:30

History, 22.06.2019 06:30

History, 22.06.2019 06:30

Biology, 22.06.2019 06:30

History, 22.06.2019 06:30

Biology, 22.06.2019 06:30


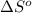 for the surrounding when given amount of HCl gas is reacted is 73.21 J/K
for the surrounding when given amount of HCl gas is reacted is 73.21 J/K![\Delta S^o_{rxn}=\sum [n\times \Delta S^o_{(product)}]-\sum [n\times \Delta S^o_{(reactant)}]](/tpl/images/0536/8507/52737.png)
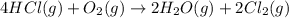
![\Delta S^o_{rxn}=[(2\times \Delta S^o_{(Cl_2(g))})+(2\times \Delta S^o_{(H_2O(g))})]-[(4\times \Delta S^o_{(HCl(g))})+(1\times \Delta S^o_{(O_2(g))})]](/tpl/images/0536/8507/2475f.png)
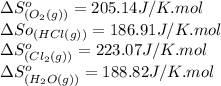
![\Delta S^o_{rxn}=[(2\times (223.07))+(2\times (188.82))]-[(4\times (186.91))+(1\times (205.14))]\\\\\Delta S^o_{rxn}=-129J/K](/tpl/images/0536/8507/8e27e.png)