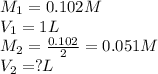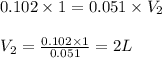Chemistry, 07.03.2020 00:59, jerikkaharris7057
You make 1.000 L of an aqueous solution that contains 35.0 g of sucrose (C12H22O11).
A) What is the molarity of sucrose in this solution? (I got 0.102 M for Part A) I just can't figure out part B.
B) How many liters of water would you have to add to this solution to reduce the molarity you calculated in Part A by a factor of two?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, amandasantiago2001
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 11:30, ayoismeisjjjjuan
Which statement best describes the flow of energy in this scenario
Answers: 1

Do you know the correct answer?
You make 1.000 L of an aqueous solution that contains 35.0 g of sucrose (C12H22O11).
A)...
A)...
Questions in other subjects:





Mathematics, 17.07.2019 10:20






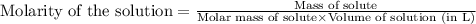
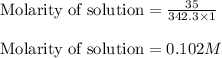
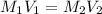
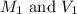 are the molarity and volume of the concentrated sucrose solution
are the molarity and volume of the concentrated sucrose solution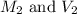 are the molarity and volume of diluted sucrose solution
are the molarity and volume of diluted sucrose solution