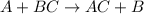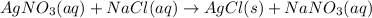Chemistry, 07.03.2020 00:36, xxaurorabluexx
The reaction AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq) is a(n) reaction.
a.
oxidation-reduction
b.
none of these
c.
single-replacement
d.
acid-base
e.
precipitation

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:10, 00015746
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 19:50, ellycleland16
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1


Chemistry, 23.06.2019 07:00, phancharamachasm
Determine the length of the object shown. 97.8 mm 97.80 mm 97 mm 98 mm
Answers: 1
Do you know the correct answer?
The reaction AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq) is a(n) reaction.
a.
oxid...
a.
oxid...
Questions in other subjects:



Physics, 22.12.2021 22:50

Mathematics, 22.12.2021 22:50


Social Studies, 22.12.2021 22:50