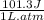Chemistry, 07.03.2020 00:09, makennskyee7114
Consider an ideal gas enclosed in a 1.00 L container at an internal pressure of 10.0 atm. Calculate the work, w , if the gas expands against a constant external pressure of 1.00 atm to a final volume of 10.0 L.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:50, britotellerialuis
Evaluate this exponential expression,8. (2 + 3)2 – 42
Answers: 3

Chemistry, 22.06.2019 07:30, zamirareece17
1. list three scientific reasons cockroaches may fly.
Answers: 1

Chemistry, 22.06.2019 08:00, hdjsjfjruejchhehd
Define dew point. i am writing this part to be able to ask the question
Answers: 1
Do you know the correct answer?
Consider an ideal gas enclosed in a 1.00 L container at an internal pressure of 10.0 atm. Calculate...
Questions in other subjects:



English, 05.09.2020 17:01




Chemistry, 05.09.2020 17:01


Physics, 05.09.2020 17:01

Mathematics, 05.09.2020 17:01