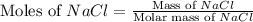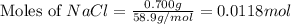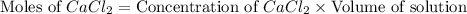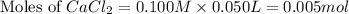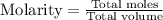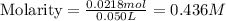Chemistry, 07.03.2020 00:21, dwilburn01
A solution is prepared by adding 0.700 g of solid NaClNaCl to 50.0 mL of 0.100 M CaCl2CaCl2. What is the molarity of chloride ion in the final solution? Assume that the volume of the final solution is 50.0 mL.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:50, kukisbae
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1

Chemistry, 22.06.2019 06:30, khalaflaf2684
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1


Chemistry, 22.06.2019 22:30, brianna5626
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1
Do you know the correct answer?
A solution is prepared by adding 0.700 g of solid NaClNaCl to 50.0 mL of 0.100 M CaCl2CaCl2. What is...
Questions in other subjects:



Mathematics, 17.10.2019 04:10

English, 17.10.2019 04:10



Geography, 17.10.2019 04:10

English, 17.10.2019 04:10

History, 17.10.2019 04:10


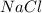 and
and 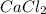 .
.