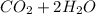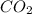Go to the Molecules mode by selecting the icon at the bottom. Notice that there are three real reactions: the synthesis of water, the synthesis of ammonia, and the combustion of methane. 1)Select each of the reactions and observe the reactants taking part in the reactions. 2)Suppose you are carrying out each of these reactions starting with five moles of each reactant, observe that some reactants are in excess whereas some reactants limit the amount of products formed. 3)Classify each of the reactants as a limiting reactant or an excess reactant for a reaction starting with five moles of each reactant. 4)Drag the appropriate items to their respective bins. View Available Hint(s) ResetHelp.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:00, hannah5143
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 22.06.2019 23:30, shukriabdisabrie
Match each statement with the state of matter it describes
Answers: 3

Chemistry, 23.06.2019 00:30, danielmartinez024m
Maya wrote if you step to describe how carbon circulates between the atmosphere and living organisms
Answers: 1
Do you know the correct answer?
Go to the Molecules mode by selecting the icon at the bottom. Notice that there are three real react...
Questions in other subjects:

Mathematics, 07.12.2021 19:10



Mathematics, 07.12.2021 19:10

Mathematics, 07.12.2021 19:10

Mathematics, 07.12.2021 19:10

Mathematics, 07.12.2021 19:10




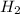 is limiting reactant ;
is limiting reactant ; 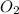 is excess reactant
is excess reactant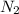 is excess reactant
is excess reactant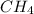 is excess reactant
is excess reactant 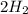 +
+ 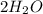
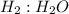
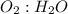
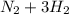 →
→ 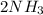
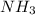
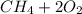 →
→