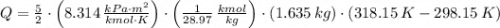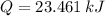Chemistry, 06.03.2020 23:46, reggiegilbert1995
A tank of 0.1m3 volume contains air at 25∘C and 101.33 kPa. The tank is connected to a compressed-air line which supplies air at the constant conditions of 45∘C and 1500 kPa. A valve in the line is cracked so that air flows slowly into the tank until the pressure equals the line pressure. If the process occurs slowly enough that the temperature in the tank remains at 25∘C, how much heat is lost from the tank? Assume air to be an ideal gas for which CP=(7/2)R and CV=(5/2)R.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:10, danielahchf
How is 0.00235 expressed in proper scientific notation? a. 2.35 × 10-3 b. 0.235 × 10-2 c. 2.35 d. 2.35 × 103
Answers: 1

Chemistry, 22.06.2019 09:00, wkalpakchi
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 09:20, UsedForSchool2018
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 12:00, Alexislol7908
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1
Do you know the correct answer?
A tank of 0.1m3 volume contains air at 25∘C and 101.33 kPa. The tank is connected to a compressed-ai...
Questions in other subjects:








History, 13.05.2020 06:57

Physics, 13.05.2020 06:57

Chemistry, 13.05.2020 06:57


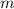 ), in kilograms, within the tank can be found by using the following form:
), in kilograms, within the tank can be found by using the following form: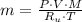 (1)
(1)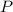 - Pressure, in kilopascals.
- Pressure, in kilopascals. 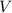 - Volume, in cubic meters.
- Volume, in cubic meters.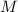 - Molar mass, in kilomoles per kilogram.
- Molar mass, in kilomoles per kilogram.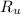 - Ideal gas constant, in kilopascal-cubic meters per kilomole-Kelvin
- Ideal gas constant, in kilopascal-cubic meters per kilomole-Kelvin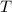 - Temperature, in Kelvin.
- Temperature, in Kelvin.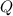 ) occur in a isochoric process, that is, a process at constant volume, it is caused by the heat released by the air flowing to the tank. The formula is represented by the following application of the definition of sensible heat:
) occur in a isochoric process, that is, a process at constant volume, it is caused by the heat released by the air flowing to the tank. The formula is represented by the following application of the definition of sensible heat: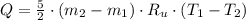 (2)
(2)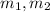 - Initial and final masses of the air within the tank, in kilograms.
- Initial and final masses of the air within the tank, in kilograms.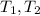 - Initial and final temperatures of the air inflow, in Kelvin.
- Initial and final temperatures of the air inflow, in Kelvin.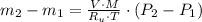 (3)
(3)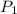 and
and 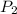 are the initial and final pressures of the air inside the tank, in kilopascals.
are the initial and final pressures of the air inside the tank, in kilopascals. 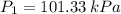 ,
, 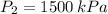 ,
, 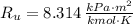 ,
, 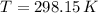 ,
, 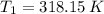 ,
, 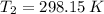 ,
,  and
and 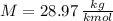 , then the heat losses are:
, then the heat losses are:![m_{2} - m_{1} = \left[\frac{(0.1\,m^{3})\cdot \left(28.97\,\frac{kg}{kmol} \right)}{\left(8.314\,\frac{kPa\cdot m^{2}}{kmol\cdot K} \right)\cdot (298.15\,K)} \right]\cdot (1500\,kPa-101.325\,kPa)](/tpl/images/0536/5617/f9891.png)