Chemistry, 17.09.2019 06:00, markitakimbrough69
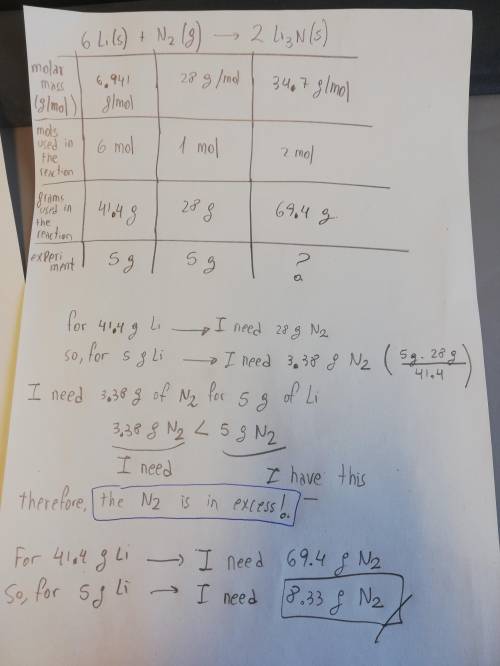
Lithium and nitrogen react in a combination reaction to produce lithium nitride: . 6li(s)+n2(g)→2li3n(s). in a particular experiment, 5.00-g samples of each reagent are reacted. the theoretical yield of lithium nitride is g.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, milkshakegrande101
Arock can be broken down into different kinds of substances by physical processes. no chemical reactions are needed to separate different parts of a rock into pure substances. this is because a rock is a(n)
Answers: 1

Chemistry, 22.06.2019 02:00, issachickadi
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Do you know the correct answer?
Lithium and nitrogen react in a combination reaction to produce lithium nitride: . 6li(s)+n2(g)→2li3...
Questions in other subjects:


English, 25.03.2021 15:50

English, 25.03.2021 15:50


Biology, 25.03.2021 15:50